Antibiotic Induced Liver Injury in a 16 year old male
1Sofia-Andriani Theodorelou-Charitou, 1Stella Kapnisi, 1Alexandros Hadjivasilis, 1Ioanna Poupoutsi, 1Anastasia Kouklidou, 1Andriana Vasilakou, 1,2George S. Potamitis
1Medical School, European University Cyprus, Nicosia, Cyprus
2Potamitis Gastroenterology- Nutrition Centre, Nicosia, Cyprus
Conflicts of interest and source of funding: none declared
ABSTRACT
A previously healthy 16 year old Caucasian male presented to the GP with flu-like symptoms. After the patient has been treated with Azithromycin and Co-Amoxiclav, he developed deteriorating symptoms that resembled liver failure. After the conduction of the appropriate screening tests, the diagnosis of drug induced liver injury was established and the patient underwent liver transplantation. Many different case reports have been published suggesting antibiotics can cause liver injury mainly in older patients. This case report illustrates the importance of early diagnosis and management of a young patient presenting with drug induced liver injury symptoms.
Key Words: Drug induced liver injury, Antibiotics, Azithromycin, Co-Amoxiclav
INTRODUCTION
Drug-induced liver injury (DILI) is a rare underreported and underdiagnosed adverse drug reaction with significant morbidity and mortality, accounting for at least 10-15 cases per 100000 patients [1]. Despite the small percentage of patients developing toxic effects from antimicrobials, the frequency of their use makes them culprits and they are the most common drug class implicative of DILI [2].
Azithromycin is an erythromycin derivative and belongs to a subgroup of macrolides. It is a potent, well tolerated oral antibiotic with <1% of patients discontinuing the medication because of adverse effects [3].
Co-Amoxiclav is a combination of penicillin and b-lactamase inhibitor that acts on the bacterial cell wall inducing cell lysis. It is the most frequent cause of DILI in adults; however it is rare of in children [2].
This case reports the diagnosis of drug induced liver injury in a young patient after the exclusion of other possible causes of liver failure.
CASE STUDY
A 16 year old Caucasian male presented to the General Practitioner with flu like symptoms; productive cough along with tiredness and anorexia in 2009. The primary treatment was Azithromycin along with Formoterol inhaler. After no evident improvement of symptoms, Co- Amoxiclav and Prednisolone (5mg) was proposed.
A month later he developed jaundice and he was admitted to the hospital. On physical examination bilateral lymphadenopathy in the groin was observed. Differential diagnosis included viral hepatitis, autoimmune hepatitis, biliary tract disease usually related to alcohol abuse, dose related drug reaction and drug induced liver injury. Following thorough medical investigations [Table 1] a definite diagnosis was obtained.
The patient had unremarkable past and family medical history; he did not take any drugs, he was a non-smoker, non-alcohol consuming, healthy and fit male.
Blood tests suggested elevation in bilirubin levels (Total 19mg/dl, Direct Bilirubin 18mg/dl) and liver function enzymes (AST/GOT 3582 u/l, ALT/GPT 5332 u/l, γGT 32 u/l, ALP 162 u/l) along with the development of significant coagulopathy (PT 18.7 sec, INR 1.8). Serology for Hepatitis A, B, EBV, CMV and autoantibodies (ANA, AMA) were negative. Chest and abdominal CT, as well as abdominal ultrasound, indicate small right pleural effusion, hepatomegaly along with dilatation of intrahepatic biliary ducts, oedematous gall bladder walls, lymphatic congestion of hepatic hilar node, splenomegaly (160mm), ascites, free fluid in the pouch of Douglas, and thickening of intestinal loops. Normal pancreas and kidneys were observed.
Liver enzymes activity was elevated. Furthermore, bilirubin, prothrombin time and INR were remarkably increasing. Any infectious or inflammatory cause was excluded.
The diagnosis brought by investigations was seronegative hepatitis, acute liver failure. Encephalopathy progressed to grade 3 and the patient was urgently transferred to a liver transplant centre where he underwent transplantation.
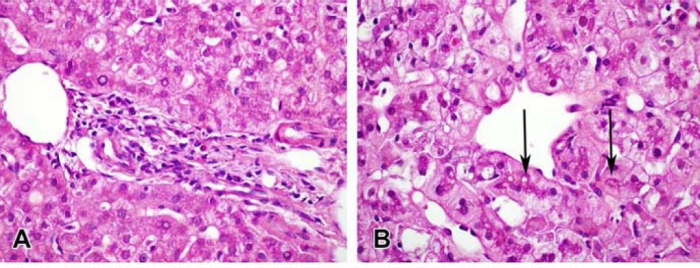
Macroscopically, a collapsed liver was observed without any evidence of regeneration. He underwent liver transplantation of an intact graft with end to end and duct to duct anastomosis.
The transplantation was successfully completed and couple of days after, along with augmented immunosuppression (Tacrolimus 3mg bd ), liver enzymes started decreasing (263 iu/l) and on ultrasound the hepatic vasculature was intact. Despite the concern of developing hypoxic brain injury along with prolonged immobility and unresponsiveness to external stimuli, he continued to have a steady progress and he was transferred from ICU to the surgical ward.
Brain MRI showed an acute cortical infarction within both superior frontal gyri in the anterior watershed zone bilaterally.
Some days after he developed significant cytopenia and a bone marrow biopsy revealed some reduction in cellularity due to Aplastic Anaemia. He was given intravenous immunoglobulin, GCSF and platelet contusion. Cytopenia was not improved, so he was still under haematological support.
A month later, he was discharged from the hospital, but he was admitted soon again with fever and rash on his face, trunk and hands associated with itching. He was given Ciprofloxacin and he was discharged. Flow cytometry established PNH.
He continued having follow up contacts with his doctors. He had an excellent prognosis and recovery as far as liver transplantation is concerned.
DISCUSSION
Drug induced liver injury is a diagnosis of exclusion as it is considered to be a rare phenomenon. Antibiotics however are one of the most common risk factors for its presentation, with Azithromycin and Co-Amoxiclav leading the way [2,4].
Drug induced liver injury can be classified as either intrinsic or idiosyncratic [5], with the first more common and dose dependant [5]. Symptoms and signs of hepatitis could develop soon after the first drug administration and can persist even after drug discontinuation, especially with drugs that cause idiosyncratic DILI such as Azithromycin and Co-Amoxiclav [5].
The average tissue half-life of a single dose of azithromycin is between 2 to 4 days. Different reported cases state that high tissue levels of the drug and its metabolites cause hepatotoxicity [3]. The majority of them report benign clinical pattern of Azithromycin related liver injury; however there have also been cases with a more severe chronic liver injury [3].
Hepatocellular injury generally is associated with re-exposure to the same drug. In this case Azithromycin was switched to co-Amoxiclav.
The Clavulanic Acid component of Co-Amoxiclav might be the major cause of liver injury, usually manifested as cholestatic hepatitis [2]. According to the literature, some patients who received Co-Amoxiclav experienced jaundice, lethargy, nausea and abdominal pain within few weeks up to 3 months [2]. Co-Amoxiclav induces DILI through three possible mechanisms. First, Clavulanic Acid might cause the formation of hepatic antigens that modify the Amoxicillin specific T cell response. Second, Clavulanic Acid specific T cells might release mediators that entrap Amoxicillin specific T cells to the liver or settle T cell mediated cytotoxicity. Finally, hepatic stress signalling pathways are stimulated by Clavulanic Acid enhancing hepatic induction of T cells [6]. Specific HLA genotypes are also associated with liver injury by Co-Amoxiclav, but more research needs to be conducted [1]. Older age, multiple prescriptions and longer duration of treatment could also play a role in the development of DILI [7].
As far as children are concerned, the clinical image is not clear as DILI is rare. It may present as an asymptomatic biochemical hepatitis or even as an acute liver failure. Causative factors have been identified as liver maturity, enzyme heterogeneity, genetic susceptibility, intensity of treatment and drug interactions [2]. Therefore Azithromycin and Co-Amoxiclav need to be carefully administered as they can induce hepatotoxicity even following a short-term therapeutic course [3].
This case report states the importance of a careful choice of antimicrobials that may cause serious damage to the liver. Emphasis should be given on further research.
REFERENCES
- Tujios S, Fontana R. Mechanisms of drug-induced liver injury: from bedside to bench. Nature Reviews Gastroenterology & Hepatology 2011;8:202-211
- Pawłowska J. Amoxicillin/clavulanic acid-induced cholestatic liver injury after pediatric liver transplantation. Annals of Transplantation 2012;17:128-131
- Lockwood A, Cole S, Rabinovich, M. Azithromycin-induced liver injury: Table 1. American Journal of Health-System Pharmacy 2010;67:810-814
- Sarges P, Steinberg J, Lewis J. Drug-Induced Liver Injury: Highlights from a Review of the 2015 Literature. Drug Safety 2016;39:801-821
- Chalasani N, Hayashi P, Bonkovsky H, Navarro V, Lee W, Fontana R. ACG Clinical Guideline: The Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. The American Journal of Gastroenterology 2014;109:950-966
- Kim S, Saide K, Farrell J et al. Characterization of amoxicillin- and clavulanic acid-specific T cells in patients with amoxicillin-clavulanate-induced liver injury. Hepatology 2015;62:887-899
- Fisher K, Vuppalanchi R, Saxena R. Drug-Induced Liver Injury. Archives of Pathology & Laboratory Medicine 2015;139:876-887